Abstract
Background: Ven+HMA is now a standard treatment (Tx) for newly diagnosed (ND) AML in patients (pts) aged ≥75 years (y), or with comorbidities precluding intensive chemotherapy. The Phase 3 VIALE-A trial demonstrated clinical benefit and longer overall survival (OS) for Ven+azacitidine (Aza) vs Aza alone; however, frequent Ven dose modifications (mods) occurred due to cytopenias (DiNardo et al. NEJM 2020). We describe real world (RW) Tx practices and outcomes in ND AML pts treated with Ven+HMA in the US.
Methods: This retrospective cohort study used the Flatiron Health electronic health record (EHR)-derived, nationwide, de-identified database. Pts aged ≥18 y with ND AML, initiating Ven+HMA Tx ≤30 days (d) from diagnosis, from Jun 1, 2018 to Jan 31, 2020, were included (i.e., prior to VIALE-A data availability, reflecting early experience). Ven Tx data and Tx mods (Ven dose and Tx schedule changes [in-cycle interruptions, cycle delays, schedule per cycle changes]) were abstracted from the EHR, including frequency of and reasons (where documented) for Tx mods and discontinuations (d/c). Timing of bone marrow (BM) biopsy and response to Tx were measured. BM response was defined as ≤5% blasts by BM biopsy. RW complete response/complete response with partial hematologic recovery (rwCR/CRh) was defined as ≤5% BM blasts, with platelet count >50 × 10 9/L and absolute neutrophil count >0.5 × 10 9/L, within 14 d of BM biopsy. Tx mods post-rwCR/CRh are described. Median Ven+HMA Tx duration, and OS from start of Ven Tx to d/c, death, or censoring at the last EHR activity before data cutoff (Aug 31, 2020) were examined by Kaplan-Meier analyses. Time-varying survival analyses assessed the effect of Ven Tx mods on Tx duration and OS.
Results: A total of 169 eligible pts treated with Ven+HMA were included. Median age at diagnosis was 77 y, 27% of pts had an ECOG performance status ≥2, 44% had secondary AML, and the overall majority (85%) were treated in community practice. European LeukemiaNet (ELN) classification was 13% favorable, 22% intermediate, 39% adverse, and 26% unknown.
By Day 7 of Tx (after ramp-up), Ven dose was 400 mg in 49% of pts, 300 mg in 3%, 200 mg in 20%, and ≤100 mg in 20%. Dose was not recorded in 8% of pts. Of 72 pts with doses <400 mg, 19 (26%) had concomitant Tx with CYP3A4 inhibitors documented in the EHR. In total, 56/169 (33%) pts had Ven dose changes in the subsequent Tx cycles, with toxicity (38%) or drug-drug interaction (25%) the most common reasons. Tx schedule changes were common and noted in 101 (60%) pts; primarily due to toxicity (78% of pts). Median time to first Tx schedule change was 33 d (95% confidence interval [CI] 28-52), at approx. 1-2 Tx cycles.
At 7.2 months (mo; range 0.6-24.8) median follow-up, median Tx duration was 5.2 mo (95% CI 4.0-7.7) and median OS (mOS) was 8.4 mo (95% CI 7.2-11.1).
Of the 95 pts with BM data during follow-up, 51 (54%) had their first biopsy by Day 28 (±14) of Tx (proxy for BM around Tx Cycle 1), with the majority of pts (41/51; 80%) achieving a BM response at that time. Of the 82% (78/95) of pts who had a BM response at any time, 47% (45/95) had a rwCR/CRh. Three of 95 (3%) pts with documented BM biopsy had early mortality (≤60 d of starting first-line Tx) vs 14/74 (19%) pts without documented BM biopsy. Tx mods post-rwCR/CRh occurred in 25/45 (56%) pts; dose holds occurred in 7/25 pts, cycle delays in 8/25, Tx schedule changes in 6/25, dose changes and d/c in ≤4/25.
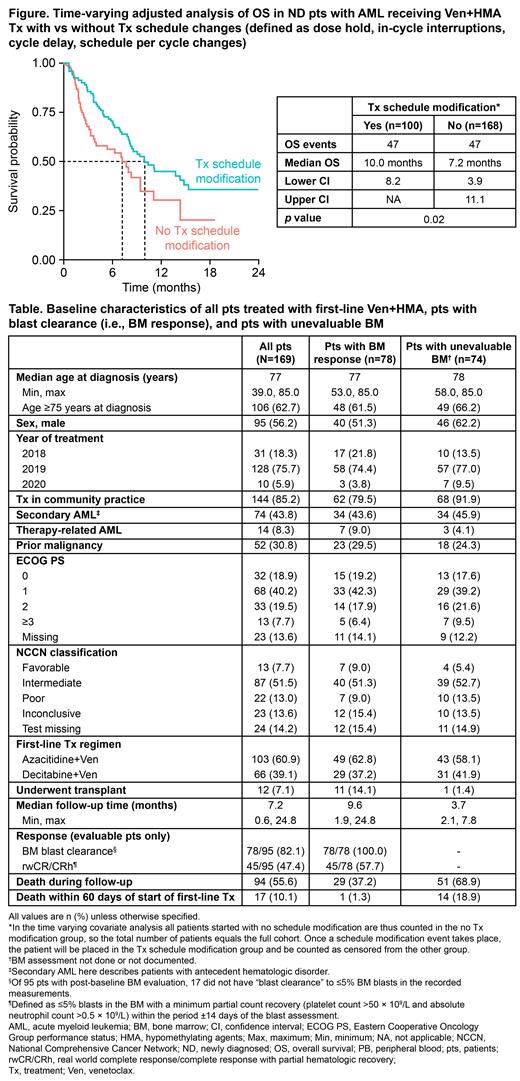
Within the entire cohort, time-varying adjusted analyses showed that, compared with pts with no Tx schedule changes, those with Tx schedule changes had a longer median Tx duration (4.2 vs 6.0 mo, respectively; non-significant) and longer mOS (7.2 vs 10.0 mo, respectively; p=0.02; Figure).
Conclusions: This study reflects early RW experience with Ven+HMA Tx in a predominantly community setting, ahead of Phase 3 VIALE-A data availability. Around half of pts started on full-dose Ven, suggesting that azole prophylaxis was either deferred or not received in many pts, although not all pts on lower doses had documented CYP3A4 inhibitor Tx. Only half of pts had a documented BM biopsy at approx. Cycle 1, but a high response rate was observed in evaluated pts. While the RW cohort reported here had a shorter follow-up time and mOS than reported in clinical trials, pts with Tx schedule mods had longer OS vs those without. These observations highlight, among other points, the importance of appropriate Ven management, including early BM assessment, to optimize pts' outcomes.
Vachhani: CTI BioPharma Corp: Consultancy; O'Neal Comprehensive Cancer Center, University of Alabama at Birmingham: Current Employment; Abbvie: Consultancy; Agios: Consultancy; Pfizer: Consultancy; Incyte: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Astellas Pharma: Speakers Bureau; Seattle Genetics: Research Funding; Blueprint Medicines: Consultancy. Abbas: Tennessee Oncology: Current Employment; Jazz: Consultancy, Speakers Bureau; TG: Consultancy, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Incyte: Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau. Flahavan: Roche Products Ltd. UK: Current Employment; Roche: Current equity holder in publicly-traded company. Ma: Genentech, Inc.: Current Employment, Other: May hold equity. Xu: F. Hoffmann-La Roche AG: Current Employment. Jin: Roche: Current equity holder in publicly-traded company; Genentech Inc: Current Employment. Montez: Genentech, Inc: Current Employment, Other: May hold equity. Huang: Genentech: Current Employment; University of Washington: Ended employment in the past 24 months. Gershon: Genentech: Current Employment; F. Hoffmann-La Roche Ltd: Current holder of stock options in a privately-held company. Ku: Genentech: Current Employment, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Roche: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Flores: Genentech: Current Employment; Roche: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Onishi: Genentech: Current Employment; Roche: Current Employment, Current equity holder in publicly-traded company. Bui: Abbvie: Current Employment, Other: May hold equity.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal